| Material | Heat capacity C MJ m-3 K-1 |
Specific heat c kJ kg-1 K-1 |
Density ρ Mg m-3 |
|---|---|---|---|
| Air | 0.0012 | 1.01 | 0.0012 |
| Water (liquid) | 4.1800 | 4.18 | 1.0000 |
| Ice | 1.9000 | 2.10 | 0.9000 |
| Mineral soil | 2.1000 | 0.80 | 2.6500 |
| Organic soil | 2.5000 | 1.90 | 1.3000 |
| Rock | 2.0000 | 0.80 | 2.7000 |
Soil Thermal Properties
Today’s learning objectives
- Provide examples of why processes in the ground are of interest to climatologists.
- Discuss key properties that describe the thermal behavior of the surfaces in the climate system.
- Explain how the key properties relate to heat conduction in soils.

Discussion (iClicker)
Why might climatologists be interested in studying soil thermal properties and subsurface processes?
Agriculture

Soil temperatures directly impact germination and growth of plants
Thermokarst
Melting ground ice, Tuktoyaktuk coastlands, 2017
Carbon Storage

Soils can act as a carbon sinks or sources
Role of Soil in the Climate System
The “active” layer of surface extends down to a relatively shallow depth.
- The properties this layer make \(\Delta S\) of sensible heat and water significant over diurnal and annual scales.
- Soils act as a ‘battery’ of both energy and mass relevant effecting the atmosphere.
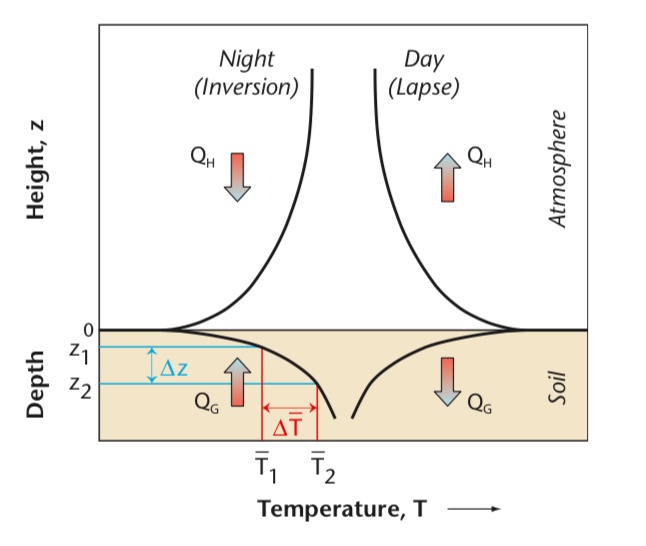
Thermal Inertia
Specific heat \(c\): the quantity of heat required to raise the temperature of a unit mass of a material by 1 K.
- Given in J kg-1 K-1.
Heat capacity \(C\) is the quantity of heat required to raise the temperature of a unit volume of a material by 1 K.
- Given in J m-3 K-1.
\(C\) and \(c\) of soil materials
Volume Fractions and Porosity
\[ \theta_a+\theta_w+\theta_g = 1 \qquad(1)\]
Where \(\theta_a\), \(\theta_w\), and \(\theta_g\) are fractional volumetric contents of soil grains, water, and air respectively. We can then define porosity \(\theta_P\) as: \[ \theta_P = \theta_a + \theta_w = 1 - \theta_g \qquad(2)\]
It is also helpful to further partition soil into mineral \(\theta_m\) and organic \(\theta_o\) fractions:
\[ \theta_g = \theta_m + \theta_o \qquad(3)\]
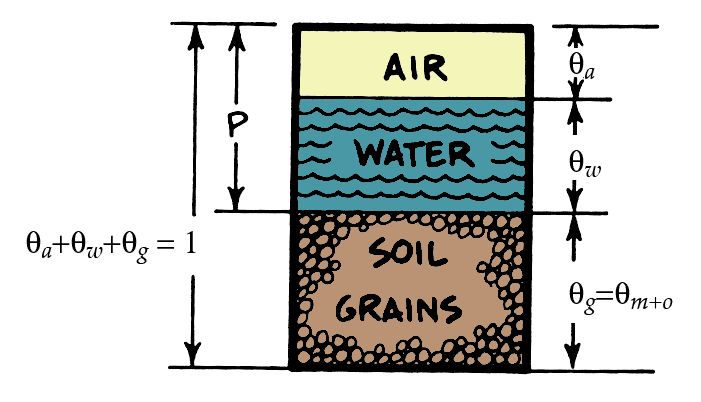
Volume Fractions and Porosity
Given a sample of know volume and mass, we can determine the fractional composition by:
- Oven-drying soil samples to get \(\theta_w\)
- Burning the dried sample in a furnace to get \(\theta_o\)
- The residual will give us \(\theta_m\)

Bulk Density
Given a “wet” soil sample of know volume (\(V\)) and mass (\(M\)), we can calculate it’s “wet bulk density” \(\rho_w = \frac{M}{V}\).
- Upon drying the sample, we can re-weigh the sample to get it’s dry mass (\(M_{dry}\)), i.e., the mass of all the soil grains.
- W can then get its “dry bulk density” \(\small\rho_{dry} = \frac{M_{dry}}{V}\), where \(V\) is fixed to the original value.
- Porosity will be inversely related to \(\rho_{dry}\)
- Higher \(\rho_{dry}\) means lower \(\theta_P\) and vice versa.
- Upon firing the sample, you can get the mass fractions of mineral \(M_m\) and organic \(M_o\) to determine the % organic content
Compound substances
The heat capacity of a mixture of substances such as soil can be calculated if the heat capacity and volume fraction of each component are known. In the case of soil:
\[ C_{s}=C_{m}\theta_{m}+C_{o}\theta_{o}+C_{w}\theta_{w}+C_{a}\theta_{a} \qquad(4)\]
Which example would have the lowest heat capacity?



Heat capacity vs. Soil Moisture
import numpy as np
import matplotlib.pyplot as plt
def get_C(theta_m,theta_o,theta_w):
C_w,C_m,C_o,C_a = 4.18, 2.1,2.5,0.0012 # MJ m-3 K-1
theta_a = 1-(theta_m+theta_o+theta_w)
theta_a[np.where(theta_a<0)]=np.nan # <<<<<< What does this line do?
C = C_m*theta_m + C_o*theta_o + C_w*theta_w + C_a*theta_a
return(C)
theta_w = np.linspace(0,1,25)
C_mineral = get_C(.5,0,theta_w)
C_mixed = get_C(.35,.2,theta_w)
C_organic = get_C(0,.1,theta_w)What does line 7 in the above codeblock do?
- A: limits \(C\) to plausible values because volumetric fractions cannot be negative
- B: limits \(C\) to plausible values because volumetric fractions cannot sum to more than one
Heat capacity and soil water content
- What is the slope of the line?
- What explains the offset between the lines?
theta_w = np.linspace(0,1,25)
plt.figure(figsize=(6,6))
plt.plot(theta_w,get_C(.5,0,theta_w),color='black',label='Mineral Soil')
plt.plot(theta_w,get_C(.35,.2,theta_w),color='blue',label='Mixed Soil')
plt.plot(theta_w,get_C(0,.1,theta_w),color='green',label='Organic Soil')
plt.ylabel(r'MJ m$^{-3}$ K$^{-1}$',fontsize=12)
plt.xlabel(r'$\theta_w$ %',fontsize=12)
plt.grid()
plt.legend(fontsize=12)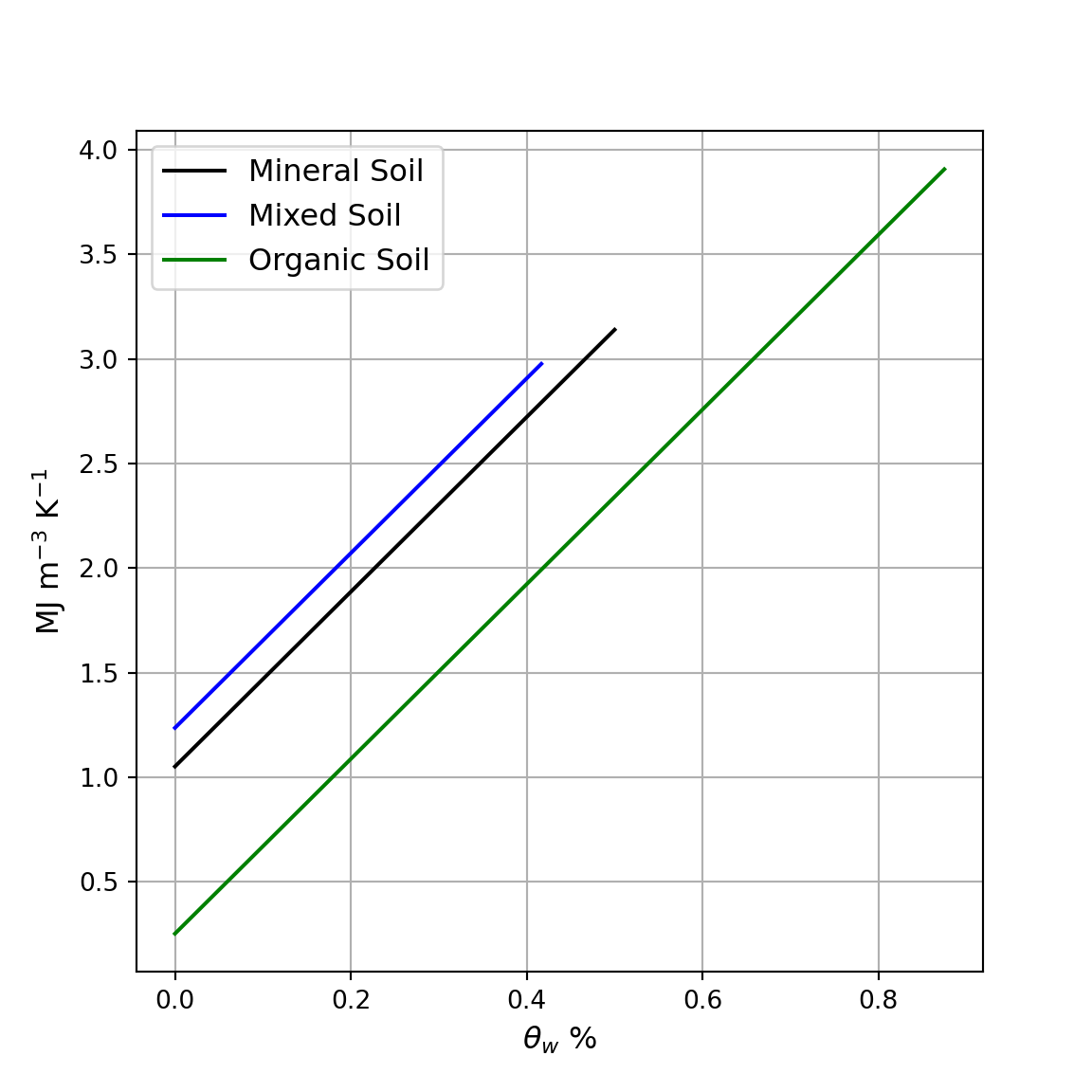
Soil Heat Flux
The flux of heat energy through a given soil layer (\(\frac{H_g}{z}\)) is a product of the layers heat capacity \(C\) and it’s rate of temperature change with time (\(\frac{\Delta T_s}{t}\)):
\[ \frac{H_g}{z} = C_{s} \frac{\Delta T_{s}}{\Delta t} \qquad(5)\]
- Why do we need to consider \(H_{g}\) on a per-layer (\(z\)) basis?
Soil Heat Flux (iClikcer)
Evaluating the above code give a value of: 0.000 W m-2 Does this appear correct?
- A True
- B False
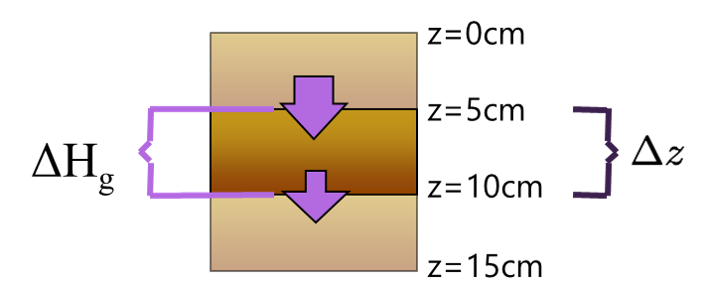
Heat Flux Divergence
If we know the heat flux at the top and bottom of some soil layer, we can calculate the change in temperature over the layer as:
\[ \small\frac{\Delta T_s}{\Delta t} = -\small\frac{1}{C_s}\small\frac{\Delta H_g}{\Delta z} \qquad(6)\]
where: \(\small\frac{\Delta H_g}{\Delta z} = \small\frac{H_{g(z_2)}-H_{g(z_1)}}{z_2-z_1}\)
- \(\Delta H_g\) is the heat flux divergence term
- It is the energy that goes into (or is released from) the layer
Fourier’s Law
Gives us the flux density of conducted heat (e.g., soil heat flux \(H_g\)) as a function of the temperature gradient and the thermal conductivity (\(k\)) in W m-1 K-1
\[ H_G = -k \small\frac{T_2 - T_1}{z_2 - z_1} \qquad(7)\]

Fourier’s Law
What is the difference between Equation 7 and Equation 5?
Thermal Conductivity \(k\)
A metric, given in W m-1 K-1, that indicates how well heat conducts through an object. Influenecd by conectivity, homogeneity, and density of the object.
- Mineral matter is a good conductor
- Water is intermediate
- Air is very poor
| Material | k W m-1 K-1 |
|---|---|
| Air | 0.025 |
| Water (liquid) | 0.590 |
| Ice | 2.100 |
| Quartz | 8.800 |
| Clay minerals | 2.900 |
| Organic matter | 0.250 |
| Stainless steel | 21.000 |
| Copper | 380.000 |
\(k\) vs. \(\theta_w\)
A non-linear relation exists between \(k\) and soil water content (\(\theta_w\))
- Adding water to dry soil (a) initially causes k to increase rapidly – rapid increase in area of contacts between soil particles resulting from water film.
- As more water (b) is added, k increases less rapidly – area of contacts increases more slowly per unit of water added (i.e. diminishing returns).


\(k\) vs. \(\theta_w\)
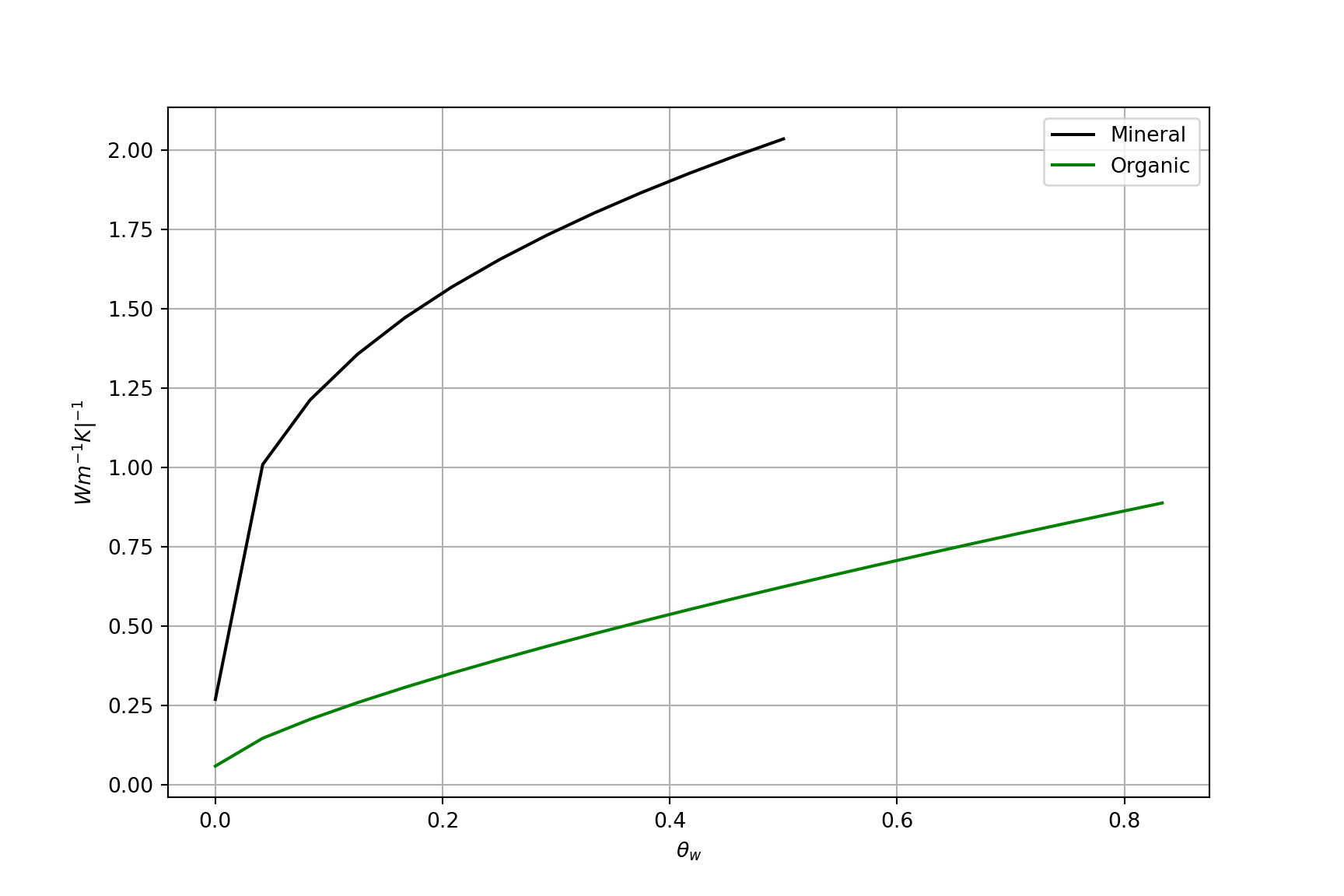
Thermal diffusivity \(k\) of soil vs. volumetric water content \(\theta_w\)
Thermal diffusivity \(K\)
Thermal diffusivity K – indicates how quickly soil at depth will warm or cool in response to heating or cooling at the surface. It tells us how fast a temperature wave will diffuse or travel downward into a soil.
- It’s units are m2 s-1 and it is defined as:
\[ K = \frac{k}{C} \qquad(8)\]
Why the curious shape?
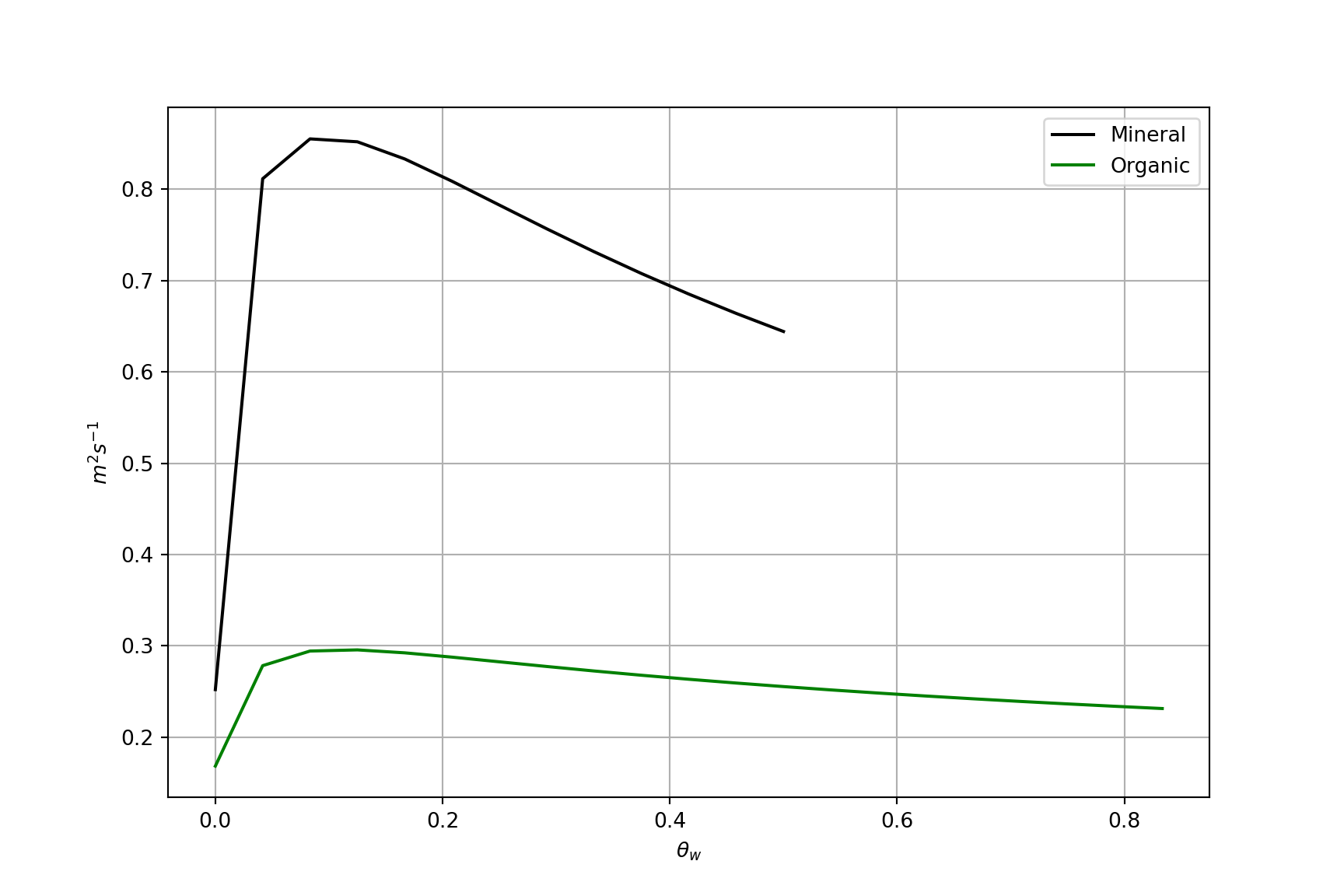
Thermal diffusivity \(k\) of soil vs. volumetric water content \(\theta_w\)
Measuring Soil Heat Flux
Theoretically, soil heat flux could be simply measured using a vertical array of thermocouples using Fourier’s law.


Measuring Soil Heat Flux
Practically, the problem is the variably \(k\) with water content. Requires continuous measurements of soil volumetric water content \(\theta_w\).
- \(\theta_w\) can be measured precisely in a lab using the gravimetric method or approximated in the field using time domain refractometry (TDR)
- TDR is an indirect measure based on the travel time of a high frequency electromagnetic pulse through the soil


Measuring Soil Heat Flux
Soil heat flux plates provide a more direct measure of \(H_g\).

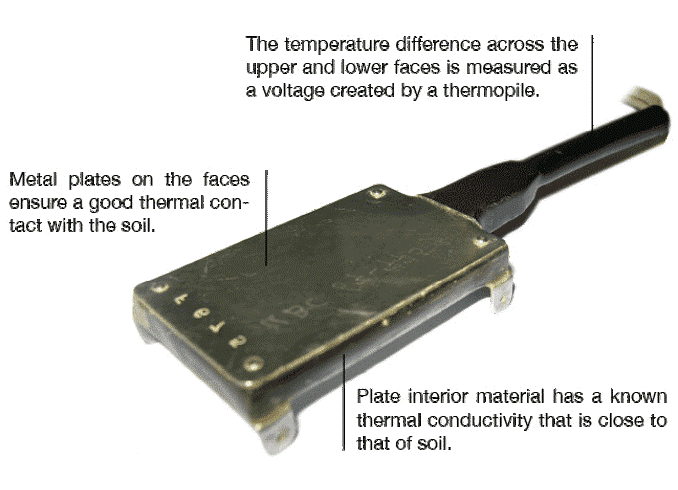
Measuring Soil Heat Flux
Soil heat flux plates provide a more direct measure of \(H_g\). The can also be used to estimate the heat capacity \(C\) and thermal conductivity \(k\) of a soil in the field following Equation 5 and Equation 7 respectively.
Estimating \(C\)
\(T_s\) at 5 and 10 cm at 12:00 are 4.18 and 1.98 \(^{\circ}C\) respectively; 1 hour later they are 4.83 and 2.45 \(^{\circ}C\) respectively. Mean \(H_g\) over the interval is 18.035 \(W m^{-2}C\). Approximate \(C\)
T_1 = (4.18+1.98)/2 # Average temperature (deg C) between 5cm and 10cm at 12:00
T_2 = (4.83+2.45)/2 # Average temperature (deg C) between 5cm and 10cm at 13:00
H_g = 18.035 # Average soi heat flux (W m-2) at 7.5 cm over the interval
Delta_T_delta_t=(T_2-T_1)/3600 # Change in temperature over the 3600 s interval
C_s = H_g/.05/Delta_T_delta_t * 1e-6 # Rearrange and solve, and convert from J to MJ
sprintf('C = %.2f MJ m-3 K-1',C_s)[1] "C = 2.32 MJ m-3 K-1"Estimating \(k\) (iClicker)
At 12:00 \(T_s\) at 5 and 10 cm are 4 and 2 \(^{\circ}C\) respectively and “instantaneous” \(H_g\) is 15 \(W m^{-2}C\). Approximate \(k\)
T_1 = 4 #C
z_1 = 5 #cm
T_2 = 2 #C
z_2 = 10 #cm
H_g = 15 #Wm-2
# Rearrange Fourier's Law
k = -(z_2-z_1)/(T_2-T_1)*H_g
sprintf('k = %.2f W m-1 K-1',k)[1] "k = 37.50 W m-1 K-1"What’s the issue here?
- A Looks correct
- B Didn’t convert from K to C
- C Didn’t convert from cm to m
Soil Thermal Admittance \(\mu\)
The ability of the soil surface to accept or release heat following a change in soil heat flux (\(H_G\)). How well it can “soak it up” without changing temperature much. It is given by:
\[ \mu = \sqrt{k C} \qquad(9)\]
- \(\mu\) is the soil thermal admittance; \(k\) is the thermal conductivity and \(C\) is the heat capacity
- Note the similarities to thermal diffusivity Equation 8
- Units: J m-2 K-1 s-1/2
Thermal Admittance and Surface Temperature
For a change in soil heat flux (i.e., \(\Delta H_G\)), the change in surface temperature (\(\Delta T_s\)) is inversely proportional to \(\mu\)
- For the same energy added:
- Soil with a low \(\mu\) warms and cools more rapidly
- Soil with a high \(\mu\) warms and cools more slowly
\[ \Delta T_s = \frac{\Delta H_g}{\mu} \qquad(10)\]
\(\mu\) vs \(\theta_w\)
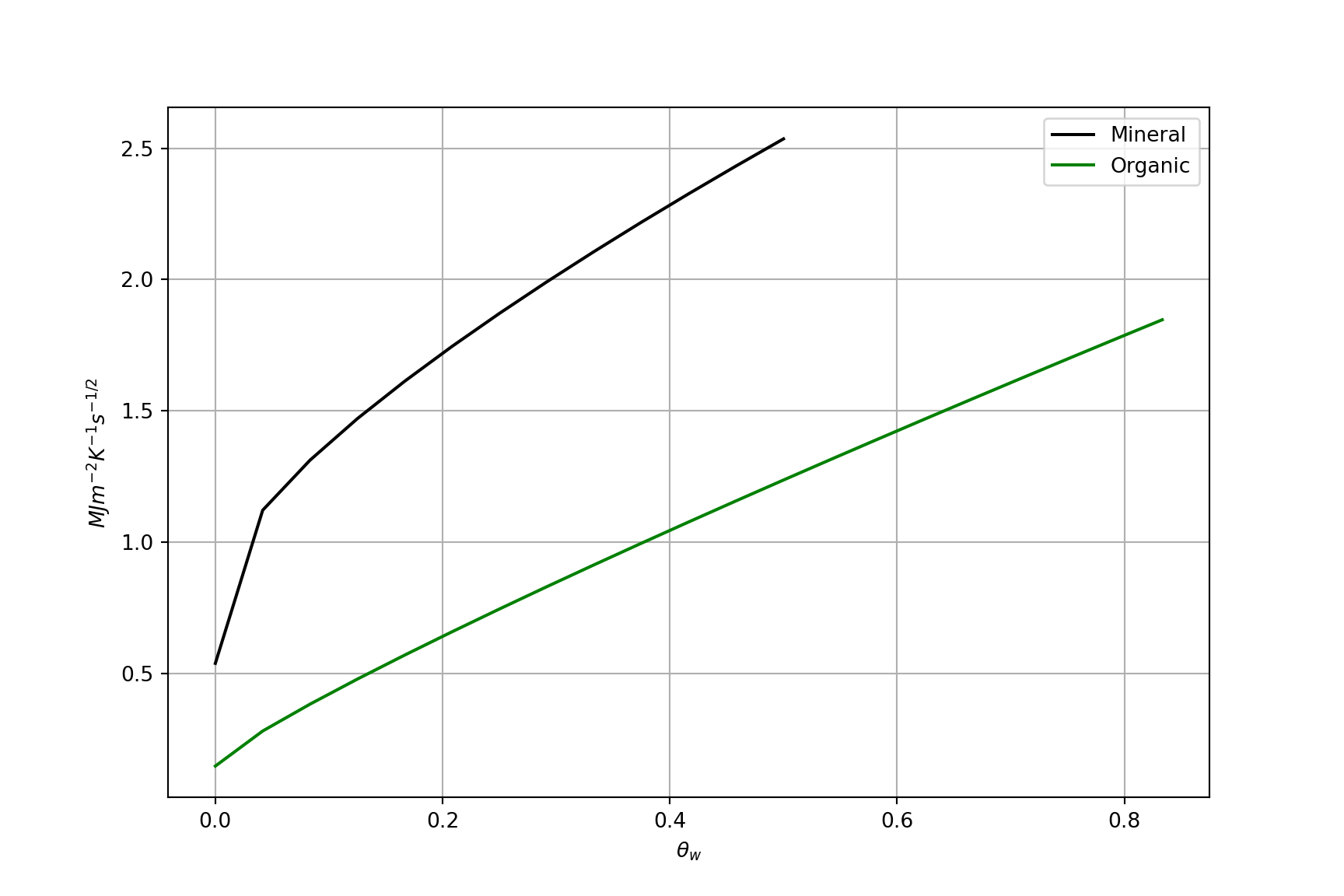
Thermal admittance \(\mu\) of soil vs. volumetric water content \(\theta_w\)
Surface temperature vs \(\theta_w\)
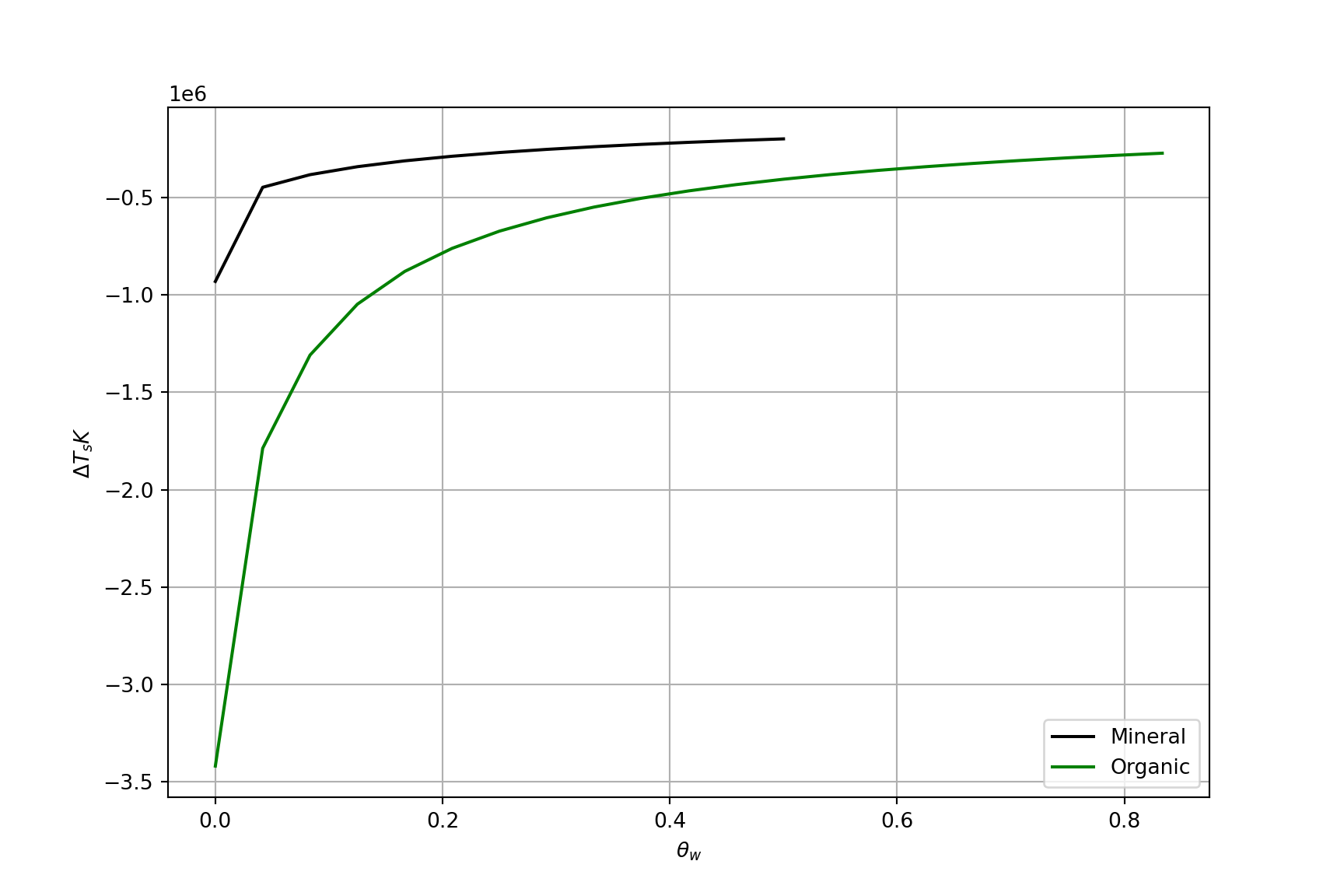
Surface temperature change vs. volumetric water content \(\theta_w\) over a night with 0.5 MJ radiative cooling
Surface temperature vs \(\theta_w\) (iClicker)
Given the plot on the previous slide, at any equivalent value of \(\theta_w\), which soil would better prevent frost damage on a calm clear night?
- A Organic
- B Mineral
Temperature Waves
The variation of temperature with depth can be thought of as waves moving down through the soil in response to radiation “forcing” at the surface.
- There are annual waves, diurnal waves, and waves with shorter periods due to clouds
- In typical mineral soils:
- Annual waves penetrate to ~ 10 m
- Diurnal waves to ~ 50 cm
- Cloud waves to ~ 5 cm
Annual Waves
Mean soil temperatures by month in Burns Bog
Shorter Timescale Waves
Soil temperatures in Burns Bog, June 2023
Summarzign Thermal Properties
How rapidly does a volume warm when a certain amount of energy is supplied?
- Heat Capacity \(C\)
How well does heat conduct from one depth to another for a given temperature gradient?
- Thermal conductivity \(k\)
How rapidly does a soil warm at depth if energy is available at the surface?
- Thermal diffusivity \(K\)
Take home points
- Soils are important for storage of heat and water in the climate system.
- Two basic thermal properties regulate the exchange - Heat capacity Cs and thermal conductivity k. From those we can derive thermal diffusivity κ = k / Cs.
- The water content of the soil is significantly altering both C (linearly) and k (non-linearly).